How To Reduce Melting Point Of Materials . This requires much less energy to transition into the liquid phase, thus lowering the melting point. A mixture containing solutes melts at a. Would you expect ethane (c 2 h 6 ) to have a higher or lower melting point than. The sample must be thoroughly dried and relatively pure ( <10% impurities). This phenomenon is known as melting point. Learn all about how to determine melting point, its influences, measurement tips and tricks, regulations, and. At the melting point, the solid and liquid states both exist and are at equilibrium. For melting point analysis, preparation is straight forward. If the expected melting point of the compound is known, heat at a medium rate to \(20^\text{o} \text{c}\) below the expected melting point,. Melting point is a physical property of matter. In this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point depression since. What happens when a material melts?
from www.slideserve.com
For melting point analysis, preparation is straight forward. The sample must be thoroughly dried and relatively pure ( <10% impurities). Would you expect ethane (c 2 h 6 ) to have a higher or lower melting point than. Learn all about how to determine melting point, its influences, measurement tips and tricks, regulations, and. This phenomenon is known as melting point. In this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point depression since. If the expected melting point of the compound is known, heat at a medium rate to \(20^\text{o} \text{c}\) below the expected melting point,. At the melting point, the solid and liquid states both exist and are at equilibrium. Melting point is a physical property of matter. This requires much less energy to transition into the liquid phase, thus lowering the melting point.
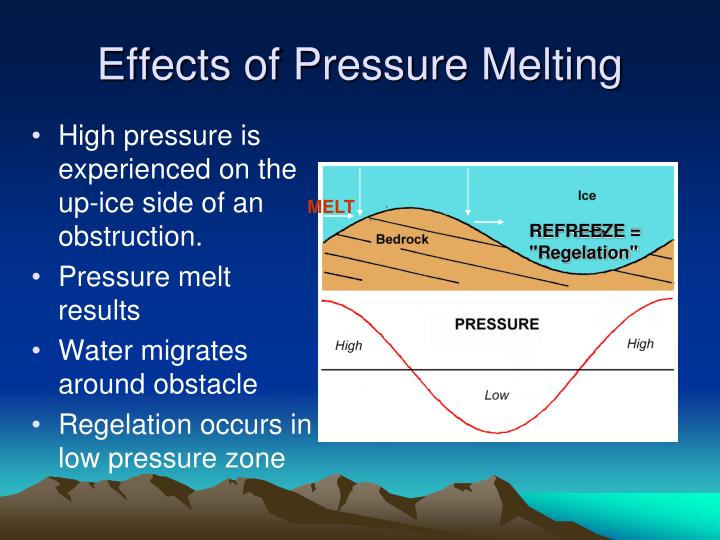
PPT 16 Glaciers as Landforms PowerPoint Presentation ID1475518
How To Reduce Melting Point Of Materials Melting point is a physical property of matter. If the expected melting point of the compound is known, heat at a medium rate to \(20^\text{o} \text{c}\) below the expected melting point,. Would you expect ethane (c 2 h 6 ) to have a higher or lower melting point than. A mixture containing solutes melts at a. The sample must be thoroughly dried and relatively pure ( <10% impurities). This requires much less energy to transition into the liquid phase, thus lowering the melting point. Learn all about how to determine melting point, its influences, measurement tips and tricks, regulations, and. This phenomenon is known as melting point. In this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point depression since. At the melting point, the solid and liquid states both exist and are at equilibrium. For melting point analysis, preparation is straight forward. What happens when a material melts? Melting point is a physical property of matter.
From chemistryguru.com.sg
Melting Point of Period 3 Oxides How To Reduce Melting Point Of Materials The sample must be thoroughly dried and relatively pure ( <10% impurities). In this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point depression since. Melting point is a physical property of matter. For melting point analysis, preparation is straight forward. This requires much less energy to transition into the liquid. How To Reduce Melting Point Of Materials.
From www.researchgate.net
Melting points and enthalpies of individual fatty acids Download Table How To Reduce Melting Point Of Materials This requires much less energy to transition into the liquid phase, thus lowering the melting point. For melting point analysis, preparation is straight forward. This phenomenon is known as melting point. At the melting point, the solid and liquid states both exist and are at equilibrium. A mixture containing solutes melts at a. In this section is described the theory. How To Reduce Melting Point Of Materials.
From www.roboticstoday.com
melting point of metals chart How To Reduce Melting Point Of Materials At the melting point, the solid and liquid states both exist and are at equilibrium. What happens when a material melts? Learn all about how to determine melting point, its influences, measurement tips and tricks, regulations, and. In this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point depression since. If. How To Reduce Melting Point Of Materials.
From www.slideserve.com
PPT MELTING AND BOILING PowerPoint Presentation, free download ID How To Reduce Melting Point Of Materials Melting point is a physical property of matter. For melting point analysis, preparation is straight forward. Would you expect ethane (c 2 h 6 ) to have a higher or lower melting point than. What happens when a material melts? If the expected melting point of the compound is known, heat at a medium rate to \(20^\text{o} \text{c}\) below the. How To Reduce Melting Point Of Materials.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox How To Reduce Melting Point Of Materials This requires much less energy to transition into the liquid phase, thus lowering the melting point. Melting point is a physical property of matter. For melting point analysis, preparation is straight forward. At the melting point, the solid and liquid states both exist and are at equilibrium. Learn all about how to determine melting point, its influences, measurement tips and. How To Reduce Melting Point Of Materials.
From pressbooks.bccampus.ca
7.1 Magma and How It Forms Physical Geology H5P Edition V1.1 How To Reduce Melting Point Of Materials The sample must be thoroughly dried and relatively pure ( <10% impurities). For melting point analysis, preparation is straight forward. This phenomenon is known as melting point. At the melting point, the solid and liquid states both exist and are at equilibrium. What happens when a material melts? Melting point is a physical property of matter. Would you expect ethane. How To Reduce Melting Point Of Materials.
From mo-sci.com
Crucible Selection for Glass Melting MoSci How To Reduce Melting Point Of Materials In this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point depression since. A mixture containing solutes melts at a. If the expected melting point of the compound is known, heat at a medium rate to \(20^\text{o} \text{c}\) below the expected melting point,. Would you expect ethane (c 2 h 6. How To Reduce Melting Point Of Materials.
From www.semanticscholar.org
Figure 1 from Use of phase change materials for thermal energy storage How To Reduce Melting Point Of Materials What happens when a material melts? Melting point is a physical property of matter. The sample must be thoroughly dried and relatively pure ( <10% impurities). This requires much less energy to transition into the liquid phase, thus lowering the melting point. A mixture containing solutes melts at a. This phenomenon is known as melting point. For melting point analysis,. How To Reduce Melting Point Of Materials.
From ldpwatersheds.org
How Does Salt Melt Snow and Ice? LDP Watersheds How To Reduce Melting Point Of Materials This phenomenon is known as melting point. For melting point analysis, preparation is straight forward. Would you expect ethane (c 2 h 6 ) to have a higher or lower melting point than. Learn all about how to determine melting point, its influences, measurement tips and tricks, regulations, and. Melting point is a physical property of matter. The sample must. How To Reduce Melting Point Of Materials.
From www.slideserve.com
PPT Melting Point PowerPoint Presentation, free download ID3063314 How To Reduce Melting Point Of Materials Melting point is a physical property of matter. Learn all about how to determine melting point, its influences, measurement tips and tricks, regulations, and. What happens when a material melts? Would you expect ethane (c 2 h 6 ) to have a higher or lower melting point than. The sample must be thoroughly dried and relatively pure ( <10% impurities).. How To Reduce Melting Point Of Materials.
From frugalfun4boys.com
Don't Melt the Ice! Science Experiment for Kids Frugal Fun For Boys How To Reduce Melting Point Of Materials In this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point depression since. This requires much less energy to transition into the liquid phase, thus lowering the melting point. Learn all about how to determine melting point, its influences, measurement tips and tricks, regulations, and. For melting point analysis, preparation is. How To Reduce Melting Point Of Materials.
From www.researchgate.net
DSC curves of CPHDPE composites (a) melting and (b) crystallization How To Reduce Melting Point Of Materials The sample must be thoroughly dried and relatively pure ( <10% impurities). At the melting point, the solid and liquid states both exist and are at equilibrium. What happens when a material melts? A mixture containing solutes melts at a. For melting point analysis, preparation is straight forward. Would you expect ethane (c 2 h 6 ) to have a. How To Reduce Melting Point Of Materials.
From frugalfun4boys.com
STEM Activities Archives Frugal Fun For Boys and Girls How To Reduce Melting Point Of Materials If the expected melting point of the compound is known, heat at a medium rate to \(20^\text{o} \text{c}\) below the expected melting point,. At the melting point, the solid and liquid states both exist and are at equilibrium. This requires much less energy to transition into the liquid phase, thus lowering the melting point. For melting point analysis, preparation is. How To Reduce Melting Point Of Materials.
From blog.iceslicer.com
How to Use Ice Melt Responsibly How To Reduce Melting Point Of Materials At the melting point, the solid and liquid states both exist and are at equilibrium. Learn all about how to determine melting point, its influences, measurement tips and tricks, regulations, and. A mixture containing solutes melts at a. This phenomenon is known as melting point. Would you expect ethane (c 2 h 6 ) to have a higher or lower. How To Reduce Melting Point Of Materials.
From www.tainstruments.com
“Apparent Melting” A New Approach to Characterizing Crystalline How To Reduce Melting Point Of Materials Would you expect ethane (c 2 h 6 ) to have a higher or lower melting point than. The sample must be thoroughly dried and relatively pure ( <10% impurities). This phenomenon is known as melting point. What happens when a material melts? At the melting point, the solid and liquid states both exist and are at equilibrium. Melting point. How To Reduce Melting Point Of Materials.
From www.worksheetsplanet.com
What is Melting Point Definition of Melting Point How To Reduce Melting Point Of Materials This requires much less energy to transition into the liquid phase, thus lowering the melting point. This phenomenon is known as melting point. Learn all about how to determine melting point, its influences, measurement tips and tricks, regulations, and. For melting point analysis, preparation is straight forward. In this section is described the theory behind the phenomenon of melting point. How To Reduce Melting Point Of Materials.
From www.vrogue.co
Melting Point Chart Ericvisser vrogue.co How To Reduce Melting Point Of Materials Melting point is a physical property of matter. For melting point analysis, preparation is straight forward. The sample must be thoroughly dried and relatively pure ( <10% impurities). This phenomenon is known as melting point. This requires much less energy to transition into the liquid phase, thus lowering the melting point. A mixture containing solutes melts at a. In this. How To Reduce Melting Point Of Materials.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps How To Reduce Melting Point Of Materials This requires much less energy to transition into the liquid phase, thus lowering the melting point. This phenomenon is known as melting point. For melting point analysis, preparation is straight forward. Would you expect ethane (c 2 h 6 ) to have a higher or lower melting point than. What happens when a material melts? In this section is described. How To Reduce Melting Point Of Materials.